Oral Microbiome: Alzheimer's, Heart Attack and Cancer Risk

Health Longevity Secrets with Dr. Robert Lufkin, MD
54 Minutes
Episode Transcript
Dr. Robert Lufkin, MD:
Welcome back to the Health Longevity Secrets Show, and I'm your host, Dr. Robert Lufkin. The oral microbiome holds clues to not only dental health, but also the risk of Alzheimer's disease, heart disease, and even cancer. Today, let's speak with an expert in that area who is helping us better understand and optimize this critically important region. Danny Grannick is CEO and co-founder at Bristle Health. They use genomics to measure the bacteria in saliva that are linked to periodontal disease and caries, and also heart attack, Alzheimer's and cancer. They then offer evidence-based recommendations of treatments for helping to prevent these diseases. Danny has a bachelor's degree in biochemistry from UCSD, and a master's in biotechnology and entrepreneurship from NYU. And now please enjoy this interview with Danny Grannick. Hey, Danny, welcome to the show.
Danny Grannick:
Hey. Thanks for having me.
Dr. Robert Lufkin, MD:
It's so great to have you here. I can't wait to hear about the interesting work you guys and gals are doing with Bristle Health and understanding the oral microbiome and the significance of that not only for dental caries and periodontal disease, but also as we're going to find out, about Alzheimer's disease and heart attack and cancer and stroke and all sorts of chronic diseases that, just a few years ago, nobody thought were associated with oral health. And so this is going to be great. But before we do that, maybe just take a moment and tell us how you got interested in this fascinating area?
Danny Grannick:
Yeah. It's been in the works for a long time. I mean, I think my background was originally in biochemistry, more on the physical chemistry side, so a lot of time in the lab. And while I was in the lab doing some research, I started to notice that I was a lot more interested in the application of the research and kind of translating it into product than I was with doing the actual research itself. So that led me into a bit of a pivot where I'd previously been looking at PhD programs, into something more on the commercial side. And I was lucky enough to start my career in the genomics industry, which at the time was really, I would say kind of crossing the chasm between this early adopter segment and becoming a really formalized technology in clinical care. So I worked at Illumina, a leader in sequencing technologies, and for background, they make a genetic sequencing technology.
Danny Grannick:
So they literally make the machine that reads your genetic code and spits out all of the different nucleotides associated with it. And they basically sell that platform technology to researchers looking into genomics or companies like 23andMe or Ancestry, along with a flurry of companies that have emerged since then investigating genomics and connecting it to health. So I got my start at Illumina and became exposed to all of these different applications and genomics. We've seen huge applications and growth in areas like oncology, non-invasive prenatal testing, obviously the gut microbiome space has gotten a lot of traction over the last couple of years, and I think it was really reflective of what we've seen across all different kinds of health technologies. We're using biometrics to collect data that previously wasn't accessible, and we're basically amassing this huge database, and we're starting to be able to drive insights from it.
Danny Grannick:
So I was at Illumina for a couple of years and had the opportunity to work with some amazing researchers and companies, and was always looking for a new area that genomics could be applied, basically what was an area in health that we had limited insights into that we could apply this increasingly democratized technology towards and uncover new insights related to health. The real story around Bristle, the specifics are that we were at a pitch competition, and I think we had heard a few companies present on the gut microbiome, and we knew that this was a space getting a lot of attention. There was still a gap between the research that was being done, and what we considered being clinically actionable results that you could provide to patients. And coincidentally, my then friend, and now co-founder had a dental appointment the next day.
Danny Grannick:
And he is, like a lot of us, your typical patient who is religious about oral hygiene, and inevitably has cavities every time he goes to the dentist, so he was kind of lamenting about what he was expecting the next day. And it kind of hit us in the face that we had never heard of a company or a research group really leveraging sequencing and genomics to focus on oral health. And we started digging into it, and basically found that there was this massive gap in our understanding of how oral diseases are manifested at the consumer and the patient level, what is implicated in as far as its connection to overall health and disease, and that a lot of these insights really reside in the oral microbiome and the data that we're able to derive.
Danny Grannick:
So the specific makeup of bacteria, fungi and viruses that live inside of our mouths are connected to a whole host of overall health factors, and we just saw this really exciting space that we could define with Bristle, and we saw a lot of utility that we could deliver to patients, and that was kind of the beginning of the company.
Dr. Robert Lufkin, MD:
Yeah, that's a great story. Well, let's start off with the microbiome. The microbiome has exploded in the public consciousness, the word microbiome, over the last few years. And many people when they hear microbiome, they equate it with the gut microbiome, but of course actually we now understand there are many microbiomes throughout the body, the sinonasal microbiome, the vaginal microbiome, the dermal microbiome, even the brain microbiome. So tell us about, what about the oral microbiome?
Danny Grannick:
Yeah. I mean, I think you bring up a really interesting pattern that we've seen, which is for a lot of clinical history, we've really thought of ourselves as individual beings, first of all, and even within our individual beings, being very compartmentalized. So we've always viewed our mouths as an individual component of our bodies, our guts as another individual component. And again, I think with a lot of the new technologies that have been surfacing, we have started to understand that, one, we aren't the individuals that we think we are, we're actually a very symbiotic ecosystem of microbes that over hundreds of thousands and millions of years have adapted to basically use our bodies to survive in, and we've also uncovered a lot of relationships between the different parts of our bodies.
Danny Grannick:
So you mentioned the gut microbiome as kind of being the star, and I think the gut microbiome was a really interesting space and still is. It was an unexplored area, but it took a while for research and I think the public to become aware that, just like there's a gut microbiome and your gut microbiome can be implicated in not only your gut health, but your overall health, we have similar microbiomes across all of our different bodies, because there's all of these different environments that lend themselves to different species of bacteria and fungi and viruses to live on. And some of those bacteria are beneficial and they help protect us from disease, others are pathogenic and may cause harm to our bodies. So there's this incredibly deep and complex layer, I think, to existing as a human being that we're just starting to unsurface.
Danny Grannick:
And the oral cavity is really interesting for us. Just like your skin, the oral cavity is one of the most exposed components of the entire body. I mean, think about how many different things get introduced to your mouth from what you eat, what you drink and what you breathe, and it's directly connected to your digestive system, to your gut, and it's a pretty critical component. So for us, the oral microbiome is a really key component of your overall health, and it's been really exciting for us to explore not only the causal relationships between pathogenic bacteria and oral disease, but pathogenic bacteria and overall disease or systemic conditions.
Danny Grannick:
It's also really fascinating... I think unlike the gut microbiome, the oral microbiome has kind of sub-environments that we're starting to investigate. So you can imagine that the kinds of bacteria that primarily live on your teeth that are maybe exposed to oxygen more frequently have a lot of movement going on, have saliva kind of flowing over them, are very different than the bacteria that live underneath your gum line closer to your bloodstream, or are different than the ones that live on your tongue. And each of those bacteria play a really important role, and the makeup of those bacteria, those sub-oral microbiomes are also very closely related to specific oral diseases and your risk for overall conditions.
Dr. Robert Lufkin, MD:
Yeah. This is a fascinating area. So it's almost like all these microbiomes, the mouth included, it's sort of like a coral reef with all these different organisms and these different little relationships and niches and all. I'm wondering of the organisms that we're going to begin to talk about in a bit, is it about the numbers of the organisms, or are there any that are strictly pathogenic, in other words, if you detect this at all, it's abnormal, or is it more, you have too many of this amount or something like that?
Danny Grannick:
Yeah, that's a really good question. So I think one of the statistics that we all see associated with the gut microbiome is something like a diversity score. And I believe that generally the higher diversity you have, the healthier your gut microbiome tends to be. There's some interesting correlations there. We're still uncovering how diversity in the oral microbiome is connected to disease, but broadly speaking, you have an oral microbiome, and a subset of those microbes are going to be beneficial and help protect against disease, and you'll have different abundances of different species that fall within that category, and then on the other end of the spectrum, we'll have pathogenic microbes, and those are the ones that confer damage to your mouth, or can be implicated in systemic disease. And again, each of those species will have a relative abundance in terms of your whole oral microbiome.
Danny Grannick:
So there's been a lot of research that has shown the ratio of the total abundance of those pathogenic species versus the total abundance of the beneficial species does relate or correlate to oral health status, which totally makes sense, but then within those subsets of pathogenic bacteria, you have some that are related to the onset of something like periodontal disease, and others that are related to the onset of something like dental caries or cavities. And the way that we present our results is, we look at the comprehensive relative abundance of species that are associated with something like periodontal disease in relation to the entirety of your oral microbiome, and that score basically helps us derive relative risk for periodontal disease, and same thing with caries and bad breath.
Danny Grannick:
So I think to date, the oral microbiome in relation to oral disease has been relatively well-defined in terms of what species are pathogenic and what a high abundance of those species means, or where those thresholds are. We've had decades of research that have looked at 11 or 12 keystone pathogens that we know are causally related to those conditions. Interestingly, there are on average I think over 200 unique species of bacteria in a user's oral microbiome, at least from the data that we have so far. So looking at 11 or 12 gives you an idea of what's going on, but there's a heck of a lot more to investigate.
Danny Grannick:
And what we've started to find is that there are microbes that are beneficial and are very common to find in the oral microbiome, but if those microbes appear in other parts of your body, they actually become pathogenic. A really good example is infected endocarditis, which is a cardiovascular condition. It's relatively common. And the theory behind that is that some of the beneficial microbes and a few of the pathogenic ones implicated in oral health can enter the bloodstream, they end up in your heart or in your arteries, they cause plaque, because as we all know, especially pathogenic microbes in your mouth love to produce plaque, and they can also lead to inflammation in localized areas, so eventually that can lead to increased risk or the incidence of heart disease in the future.
Danny Grannick:
And it's exciting, and at the same time, really frightening to think that something that's beneficial in one part of your body all of a sudden becomes harmful if it's introduced somewhere else, but I think that that's a really exciting thing, because by looking at the oral microbiome and understanding if you have pathogenic bacteria X, it may be a low risk pathogenic bacteria in your oral cavity, but if that bacteria migrates somewhere else, it can cause really severe conditions, and we can help triage patients to get the monitoring or get the preventive care they need ahead of time. So to answer your question, it's a mixture of abundance and diversity, and then the kinds of species that you have in your oral microbiome, but even deeper down, those individual species can confer varying degrees of risk in your mouth or in other parts of your body.
Dr. Robert Lufkin, MD:
So for the bacterial endocarditis, the valvular disease you mentioned from a septic valve, and you were saying that would be like a more common staph or strep, relatively benign oral bacteria, but then for the disease of the blood vessels and the plaques and for ischemic heart disease, like a heart attack, a narrowing of the coronary arteries and inflammatory vessel disease, which organism is that, or which group of organisms?
Danny Grannick:
There's a few that are implicated. I can actually send you a short list after the call. I think the really interesting piece of it to me at least is that some of those organisms are pathogenic in our mouths. So they're the same microbes that cause periodontal disease, which really is an inflammatory condition, and it makes sense that if those pathogenic microbes appear in other areas of the body, they're still going to cause inflammation. And the really interesting piece is that some of them are actually beneficial in your mouth, but can cause problems in other parts of your body. So beneficial microbes in our mouth do produce not necessarily a plaque as we traditionally look at it, but a biofilm that helps protect your teeth, but you can imagine that that biofilm in other parts of your body may cause issues, so narrowing of the valves or clogging of the arteries.
Danny Grannick:
Those beneficial microbes may be identified in other parts of your body as being pathogenic, where normally they would be identified as being beneficial in your mouth, so you're getting inflammation associated with those. It's really interesting. I think that there's even a few beneficial microbes in the oral cavity that have been associated with colorectal cancer, because they've basically migrated through your body, particularly in immunocompromised patients, and end up causing damage there.
Dr. Robert Lufkin, MD:
Yeah. Yeah. And you hit on a key point here that we're all struggling with, is the fact that they're associated with these diseases, in other words, the arrow of causality, so to speak, what's causing it, in other words, whether the bacterium causes the associated chronic disease, or something else causes the bacterium to exist there in an opportunistic fashion, sort of like if you have a leaky roof, you have mold on the floor maybe, but it's not like the mold caused the roof to leak, it's just it grows there. Maybe talk about... P. gingivalis we hear a lot, it's gotten a lot of publicity as one organism ranging all the way in the blood vessels of the coronary arteries to the brains of Alzheimer's patients, this weird association, and then its presence in the mouth. What other chronic diseases is it associated with? You mentioned cancer, some maybe pancreatic cancer also, I think, right?
Danny Grannick:
Yeah. P. gingivalis has been associated with a variety of conditions. So I believe it's been implicated in diabetes, rheumatoid arthritis. So again, looking at kind of inflammation as maybe the underlying factor between all of those. Infective endocarditis. I think that there's been also some associations just with hypertension, and then obviously Alzheimer's has kind of come to the forefront, which happened rapidly as being one of the stronger associations between oral health status and mental health. And I think it's also been interesting to see an evolution. The relationship between oral health as kind of a, let's call it a phenotypical characterization, and overall health, has been pretty recognized for decades. I'm sure if you ask a lot of the dentists that are probably listening to this conversation, you'll find that there have been noticeable patterns and associations between patients that have been diagnosed with diabetes, and higher prevalence of periodontal disease.
Danny Grannick:
And I think the really exciting thing is that we're moving past these clinical findings into mechanistically understanding what the relationship is between the oral microbiome, which is a component of oral health status, and overall health or systemic disease, and we're finding that the microbiome is really kind of that connecting link between the two. So taking P. gingivalis as kind of the prime example, P. gingivalis has been, I think, labeled a pathogen, meaning that it's kind of the worst and the most common species for periodontal disease, and there have been noted associations between the emergence of periodontal disease and the worsening of the condition, and cognitive decline. And to your point around this chicken or the egg question, people had been wondering is it cognitive decline that happens first, and maybe patients get lower adherence with oral hygiene, which puts them at higher risk for periodontal disease, is it that periodontal disease maybe somehow influences the onset of cognitive decline, and there are a lot of questions about which was coming first, and if they were truly kind of related at all.
Danny Grannick:
And there was a paper that came out... The one that I'm referring to is 2019. I'm sure that there were earlier studies that had looked at the brains of Alzheimer's patients, and at the cerebral spinal fluid. And they actually identified via genomic sequencing the presence of P. gingivalis in both the brains and CSF of these patients in question. And in particular, they had identified a byproduct of P. gingivalis called gingipains, which is a protein that P. gingivalis produces, and associated that with some hallmark biomarkers of Alzheimer's. So the emergence of two proteins in particular, amyloid beta and tau.
Danny Grannick:
And a few more studies occurred that basically was able to correlate that these patients that have P. gingivalis, the P. gingivalis enters into the bloodstream, enters into the body, ends up crossing the blood brain barrier, where it then produces gingipains in the brain, and these gingipains are able to break down the connections between your neurons and also cause some damage inside, and that leads to increased abundances of these two kind of hallmark biomarkers in Alzheimer's patients, amyloid beta and tau proteins, which I think is just a fantastic example of taking something that you observe in a clinic or really in nature, and bringing it all the way through to identifying a mechanistic relationship. And we've started to see exciting results from that research. There have actually been two companies that immediately come to mind that are developing therapeutics against P. gingivalis for this reason. Cortexyme is one, and they released some really exciting data a few weeks ago, and then the second one is a company called Keystone Bio, which is really focused on P. gingivalis itself.
Dr. Robert Lufkin, MD:
Are these two companies, are they approaching targeting P. gingivalis in the mouth, or is it [inaudible 00:25:13] delivery, or can you talk about that a little bit?
Danny Grannick:
Yeah. So I believe both companies are delivered orally. My understanding is that Cortexyme design their therapeutic to selectively target gingipains, so that protein that's by P. gingivalis, whereas Keystone Bio is looking to target P. gingivalis itself and kind of block the production of gingipains downstream.
Dr. Robert Lufkin, MD:
Oh, interesting. Yeah. And the whole idea that you mentioned, the whole association with P. gingivalis and periodontal disease, not only with Alzheimer's, but with all these other seemingly unrelated chronic diseases, but actually of course related at a fundamental metabolic inflammatory level, hypertension, diabetes, stroke, heart attack, and the cancers, they're all really linked, and even so much so that periodontal disease is used now in some studies in longevity research as an independent marker for aging, as an independent biologic clock, and it's in fact one of the priority targets for studying rapamycin, which is an mTOR inhibitor we've talked about on the show before, but applying rapamycin in humans, one of the biomarkers they want to look at is periodontal disease. And actually rolling it back on that, I'm wondering with periodontal disease, is there a reliable, consistent measure of periodontal disease? I think we've all had the experience of going into the dentist office, and they do manually probing, and then writing down the scores. It seems like that can be a little operator dependent a little bit like that, but are you aware of biomarkers for periodontal disease that might be useful?
Danny Grannick:
Yeah. I mean, I think that you're touching on a really exciting shift that we're trying to progress with Bristle. So now we're kind of moving into oral health versus what we deem as dental care. And traditionally, just like you said, the way that we characterize and diagnose dental disease has been largely observational and based on the presence, and then the severity of physical characteristics. So for periodontal disease, we use things like probing where you measure the pocket depth or the space between your tooth and gum as an understanding and diagnosis of what level of periodontal disease you have. So the deeper the pocket is, the worst the condition is. And that has been the standard for characterizing and diagnosing periodontal disease forever.
Danny Grannick:
And again, what we're trying to do with the oral microbiome is, redefine what diagnosis and characterization of disease means, moving away from using physical symptoms as kind of this marker of disease, to the specific kinds of pathogenic bacteria and their relative abundances that you have. And the advantage there is that, if you're just using physical symptoms, you already have progressed disease. You're not going to be diagnosed until those symptoms have manifested and somebody can catch them. The advantage to using molecular methods for diagnosis and sequencing is, we can detect those bacteria at the lowest abundances, oftentimes before physical symptoms have emerged, and we can also get higher resolution on what exactly is contributing to your disease.
Danny Grannick:
So it's not going to be periodontal disease as a condition, it would be P. gingivalis-induced or driven periodontal disease, and I think that there's going to be a really exciting shift in oral care in terms of what your diagnosis looks like the next time you go to a dentist. It's been tough. I mean, I think it's one of the bigger challenges that we have, is educating people around exactly what the results mean in the context of today's care, but we've seen a similar shift with oncology moving from location-based characterization of tumors to mutation-based, and I think that we'll see a similar thing with oral care.
Dr. Robert Lufkin, MD:
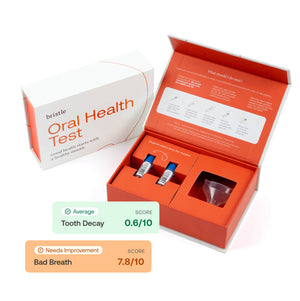
It's almost like you're the... And we'll talk about Bristle here in a moment, but the company produces... And this is a consumer-facing product as well, but produces a report of the relative concentrations of the two groups of pathogenic bacteria that you've identified for periodontal disease, and then for dental caries. And it's really beautiful, but it's almost for a biological clock as well for longevity research. It could help people plan their longevity and how that's looking as well.
Danny Grannick:
Yeah. And I think kind of to your point around using periodontal disease as a longevity marker, it's been a good start using traditional methods of characterization to make these correlations, but without the depth and the resolution that you're getting from having that molecular component, I think that there's going to be a lot of gaps in, again, understanding is this a correlation, or are they truly mechanistically related to each other.
Dr. Robert Lufkin, MD:
Maybe we could take a moment and dive into the weeds just a tiny bit. We were talking earlier, I love the way you described the approach to genetic sequencing that you use at Bristle Health, specifically qPCR versus 16S, versus shotgun metagenomics, but I think maybe you could just touch on those briefly, and it'll help people understand the power that we'll have with Bristle Health?
Danny Grannick:
Yeah. So I'll start by setting the stage with what the oral microbiome looks like. The oral microbiome at the highest level is really made up of a wide variety of bacteria, fungi and viruses. So there's essentially three different buckets of microbes that all reside in there and interact with each other in various ways to drive health or disease outcomes. Specifically looking at bacteria, to date, we have identified around 700 species that are part of the oral microbiome. And obviously the makeup of what species you have and how much abundance varies from person to person, but if we're looking kind of across the globe, there have been 700 ish species that we've found. On average, every person that we found in our data and that research has kind of uncovered has usually between 200 to 250 unique species that can be some makeup of beneficial or pathogenic bacteria.
Danny Grannick:
And as I alluded to earlier, a lot of the early research in oral health and the microbiome had really centered around these 10 to 12 species of bacteria that have been causally related to oral disease. So you can imagine that there's a set of bacteria that are causally related to periodontal disease, like P. gingivalis, there's a set of bacteria that are causally related to dental caries. So when we look at the different methods for analyzing the oral microbiome, we're going to go in the opposite direction, but qPCR is one of the most common methods, and that's been the backbone of a lot of oral microbiome research over the last 20 or 30 years. And the way that qPCR works is, you design primers that are specifically meant to... They're almost like fishing hooks that are designed for a specific kind of fish you're trying to catch.
Danny Grannick:
So qPCR, you would design primers, and the primers are meant to detect the presence or absence and the relative abundance of predetermined bacterial species. So when we look at those 10 to 12 bacteria, a lot of those tests were only testing for those bacteria. If it was there, it would be detected, and you would know around how much of it there was, if it wasn't there, it wouldn't be detected. And that's great when you're just focused on oral disease, but the problem is that you have 10 to 12 targets for what really is an environment that contains 200 potential species of different bacteria. So there was this almost black box. You don't know what you don't know. So if you're only looking for 10 or 12 species, and you find five of them, you're completely overlooking the potentially 195 other species that are present in the sample.
Danny Grannick:
So that was kind of the first method. And then with sequencing, there was [inaudible 00:35:20] that was developed, and it's called 16S. And 16S is a genomic region in bacteria, and it's a conserved region. So you can design primers that kind of stick to this gene, and then within that gene, there's a huge amount of variability across species. So it's almost like a fingerprint for bacteria. And the great thing about this method is that [inaudible 00:35:46] qPCR, you're now able to detect all of the bacteria present, and you can identify them based on that 16S sequence. So you're going from 10 to 12 kind of predetermined targets, you're missing the rest of the oral microbiome or the microbiome in general, and now we're getting all 200 bacteria usually down to the species level. So you know who's there, you have an idea of what their relative abundance is. It's been a much more comprehensive method, and again, has kind of become the most popular method when looking at the microbiome.
Danny Grannick:
The shortcomings of that are that, you're only getting the idea of the bacteria that are in the sample and really nothing else. So it's hard to derive a lot of functional information and virulence factors if you only know who's there. And then over the last five or 10 years, there's been this other method that's come up called shotgun metagenomics. And that basically is a completely hypothesis-free approach to sequencing a sample. You're doing whole genome sequencing across whatever's present. So we're getting not only the human genomic data, but we're getting bacteria, we're getting fungi, we're getting viruses, and we're getting the whole genome of those microorganisms.
Danny Grannick:
The benefit there is... I touched on being able to detect all 200 bacterial species using 16S. Well, what about the fungi and viruses that we've been overlooking the entire time? I mean, they play a huge role in health and disease. We know HPV is a really important biomarker for a variety of conditions, and shotgun metogenomics bridges that gap. So we're getting all of the bacteria, plus the fungi and viruses. And because we're getting whole genome sequence information, we can characterize those microorganisms at a deeper resolution, so moving from species level identification to strain level.
Danny Grannick:
And we're also getting all of the functional information associated with those microbes, and we can start to understand how those different microbes are interacting with each other to drive disease. Because another really important component of oral disease is, it is not a one microbe to one indication relationship, a lot of these diseases are community-driven, and we have the entire picture of what that community is to understand how they work together to drive disease. So that's what we've built our assay off of, and it's been really helpful because it produces a more accurate report as it relates to oral health and disease, but it also provides this foundation that we can start to innovate and derive new discoveries off of informing how oral health relates to overall health as well.
Dr. Robert Lufkin, MD:
Wow, that's so exciting, the ability to integrate all those different genomic types beyond just the bacteria with 16S in them or something, but viruses, like you say, HPV for oral cancer and other risk factors, and the fungi. We talked a little bit before about mold when we were offline, and that would be such an exciting area. And like you say, even the human genetic information that's in there for the individual. It's almost like this is an ideal deep learning application for AI, where you have massive amounts of genetic information across three different organism types, plus the host, and then putting it together, and there could be interactions in there that of course are not one-to-one, like you say, but are rather complex ones across all of those. It's such great, exciting possibilities in the future. So let's talk about Bristle Health a little bit. The consumers can do this themselves and send in their own samples, just saliva?
Danny Grannick:
Exactly. Yeah. So we have a direct-to-consumer saliva oral microbiome test. You can go online to bristle.com, and we've got a couple options. Users can sign up for a single test and really just get a snapshot of what's going on, we've got subscription models where we can send you a test every six months or every three months so that you can track your improvement over time. And we're always kind of updating our database and the results that we provide. So no matter which test you get and which results you have, it will continue to be a living and evolving creature, kind of like our oral microbiomes. But I mean, I think the really exciting thing and what we really want to see our users do is use the test to drive behavioral and clinical actions and changes in their lives, and really see the outcomes of those decisions and those changes in improving their oral health status and their oral microbiome.
Dr. Robert Lufkin, MD:
And right now, it's surprisingly affordable. I don't know, what's the latest pricing on that for an individual one?
Danny Grannick:
Right now, it's $119 for a single test, and then it drops to $109 per test for a test every six months, and then it drops again to $99 a test for a test every three months. We certainly hope to continue driving down the cost. I think making our test as accessible as possible is a core value of the company. And I think the unfortunate reality is that, just like chronic disease, a lot of certain socioeconomic groups are at much higher risk for oral disease and have much higher restrictions on their ability to access consistent care, so we see this as a really good way to kind of shift where the majority of oral health and care is being done into the consumer, the patient's hands and in their house.
Dr. Robert Lufkin, MD:
And I mean, ideally in a perfect world, at some point it would be wonderful if this were included with a person's dental or health insurance as part of their routine examination. Like you say, if some of these things become possible, that would be huge. I've heard you talk, or I've seen you mention things about kind of your vision for Bristle, you talked a little bit also about the notion of decentralized testing and interventions and the idea of de-health, is this de-health like DeFi for decentralized finance, which I'm a big fan of cryptocurrency as well, but talk about what is decentralized testing and decentralized health?
Danny Grannick:
Yeah. I mean, I think that there's a lot of layers to decentralization, but the exciting thing for us is, I think in medical and in dental care, but for the sake of relevance, we'll focus on dental care, it has been a very siloed industry on a number of levels. Dental practices are completely different physical locations than where you go generally to see your primary care physician. Dental insurance is a completely separate system than medical insurance, and operates almost in an inverse way where you basically have a dental insurance plan that's willing to pay up to a certain amount, and then everything afterwards is out of pocket. And as kind of an aside, we actually have the highest rate of out-of-pocket expenses in dental care in the entire healthcare system. So 40% of the $150 billion a year we spend on dental care is coming directly from patients versus what I think is around six or 8% for medical care.
Danny Grannick:
And then looking at the data, especially as we start to uncover more associations between oral health and overall health, there's a huge gap and a huge barrier in being able to get your oral health data to medical care and vice versa, which creates a lot of discrepancies and discontinuities in the care that you can get. So what we find is, because dental care is so hard to access... I think around 50% of the population doesn't see a dentist every year. And you can imagine that for a diabetic patient who may be at higher risk for oral disease and sees their endocrinologist regularly, if that endocrinologist isn't educated about oral health and the oral microbiome, those symptoms can go unaddressed, the patient doesn't get the care that they need when they need it, and that can result in really severe and prevalent oral diseases that eventually becomes an emergency that that patient then has to pay for.
Danny Grannick:
So it's kind of this vicious cycle. And I think when we talk about decentralizing health, the first response is you're trying to drive patients away from engaging with their provider. And I think it's completely the opposite. I think what we want to do is make it easier for patients to understand and act on their health status, and also make it easier to share that kind of information with the providers that they engage with. We have to meet the patient where they are, instead of trying to get them to do things that right now is really hard for them to access.
Danny Grannick:
So we're not trying to get patients to not see the dentist, it's actually quite the opposite, we're trying to go to the patients who aren't seeing dentists today, get them insights around their oral health, because they don't have it right now, and help them act on it themselves, but also facilitate a conversation with that oral healthcare professional to design personalized treatment plans or triage with their other care providers, and kind of work our way towards this idea of 360 degree health, which is a really exciting notion, but I think everybody has to remember that the patient is the one that's at the center of that circle.
Dr. Robert Lufkin, MD:
Yeah. Yeah. Well, as an expert in the oral microbiome, if you don't mind sharing with us maybe, what are your personal strategies to optimize your own oral microbiome? Just even the simple stuff as brushing, flossing, gargling. What do you do to improve that?
Danny Grannick:
Well, I'm really glad that you touched on... The simple stuff I think is the thing that stands out the most, and that's what I think is one of the most compelling messages about Bristle, Oral health is such an overlooked component in healthcare, and a lot of people don't pay a lot of attention to what they do to maintain it, and there's kind of this recurring patient experience of going to the dentist and being told to brush and floss more, but there's not an explanation of why. We know, okay, brushing helps make your teeth whiter, it cleans somehow, but it's hard to see the effect.
Danny Grannick:
And I think one of the biggest impacts that we can make as a company is, it's not getting people from level eight 8 to level 10 in terms of oral hygiene, I think it's really getting most of the population from zero to one. And getting people from zero to one will have this monumental impact on oral health outcomes, because we know that doing simple things like brushing and flossing every day have a quantitative and qualitative impact on your oral microbiome and your oral health status. We're going to be publishing some early data from our consumers, and what we've shown is... I can't remember the exact number, but the difference between people who floss daily or even every other day, and people who never floss, I think it was something like a 4x reduction in the abundance of periodontal disease-causing bacteria.
Danny Grannick:
And we all know that we should floss, but I think until you see those numbers and you really understand at a specific and quantitative level how that's improving your oral health, it's something that we don't pay much attention to. So personally for me, I got a lot better about my oral hygiene, and I think as a company, we would love nothing more than to see our users just adopt simple oral care habits that can dramatically improve their oral health, and then obviously over time we'll work on making specific changes to your diet, addressing different medications that you're taking that may be contributing to risk, but for 90% of the population and a lot of our users, it's really educating them on that zero to one and shift.
Dr. Robert Lufkin, MD:
Yeah. And just to be clear, I think... Correct me if I'm wrong, but with dental health, unlike maybe medical health where you may not see your doctor every year for a checkup, with dental health, what you're recommending the flossing and brushing and the oral microbiome and all that, it's still not a substitute for having your teeth cleaned and visiting a dentist, is that correct?
Danny Grannick:
In some ways, yeah. I mean, I think that we have this idea of cosmetic dentistry, and it's hard, because a lot of people equate dental care or cosmetic dentistry with good oral health, but they're not necessarily related. So getting a lot of dental care doesn't necessarily mean that you have good oral health all the time, whereas having good oral health almost certainly means that you are going to be able to reduce the amount of dental care that you need. So I think our goal, it's not so much telling people not to go see the dentist, but it's shifting the conversation when they do go to being, okay, well, you have a really bad cavity, you need this expensive and invasive procedure, to more of a consultative model where they're working together to improve oral health status.
Danny Grannick:
There was a... And then there's also some things with dental cleanings that we found, there was a really interesting trial, the interval trial done by the NHS, and they had shown that when you stratify the population for risk for oral disease, and you look at that low risk population, as long as they maintain good oral health and hygiene habits, there was no noticeable difference in oral health outcomes for people that got dental cleanings every six months, versus people that got dental cleanings every two years.
Danny Grannick:
So there is this really interesting idea of proactively managing your health and needing to reduce kind of the need for those in-person appointments. I am not recommending that anybody stops seeing their dentist every six months, but I do think that we'll find, again, that by improving and maintaining good oral health, the population definitely will reduce the need for traditional dental care and procedures.
Dr. Robert Lufkin, MD:
Yeah. There's so much we need to learn and we're learning, and tools like you're developing at Bristle Health will certainly play a valuable role in understanding this and having markers for assessing the things that we do. Can you tell our listeners how they can follow you on social media, or get in touch with Bristle Health? We'll put it in the show notes as well.
Danny Grannick:
Yeah. No, no, no, it's all the same. Twitter, LinkedIn, Facebook, it's all Bristle Health. So just the @ sign and Bristle Health, you'll be able to find us. We post new research and content all the time. I'd encourage at the very least going to our website and becoming a subscriber. We've got blog articles that we're constantly posting, we've got newsletters that we do every month that include the latest research in oral health in the microbiome, and then obviously getting the test gives you access to all of that kind of information at a much more personal level.
Dr. Robert Lufkin, MD:
Thanks so much, Danny, for being part of the program. And I'm going to be thinking of you later today when I floss my teeth and brush. But thanks so much for the great work you're doing, and spending an hour with us today on this program and sharing your knowledge.
Danny Grannick:
Of course. Really appreciate it.
Disclaimer:
This is for general information and educational purposes only, and it's not intended to constitute or substitute for medical advice or counseling, the practice of medicine or the provision of healthcare diagnosis or treatment, or the creation of a physician/patient or clinical relationship. The use of this information is at the own user's risk. If you find this to be of value, please hit that like button to subscribe to support the work that we do on this channel. And we take your suggestions and advice very seriously, so please let us know what you'd like to see on this channel. Thanks for watching, and we hope to see you next time.